Flexible and convenient dosing
Recommended dosing by indication
The lowest dose (1.5 mg) is a therapeutic dose as an adjunctive therapy to antidepressants for MDD1
Recommended dosing1

1.5 mg/day
Lowest and Most Commonly Prescribed Dose2*
Capsule image is not actual size.
- Day 1–Start at 1.5 mg/day
- Day 15–May increase dose to 3 mg/day
- Depending upon clinical response and tolerability, the dosage can be increased to 3 mg once daily at Day 151
- Following discontinuation of VRAYLAR, the decline in plasma concentrations of active drug and metabolites may not be immediately reflected in patients’ clinical symptoms1
- In clinical trials, dosage and titration at intervals of less than 14 days resulted in a higher incidence of adverse reactions. Maximum recommended dose is 3 mg once daily.1 (See adverse reactions from 8-week study in Section 6.1 of the full Prescribing Information)
*Data accessed October 2024.
The lowest dose (1.5 mg) is a therapeutic dose for bipolar I depression1
Bipolar I depression recommended dosing1

1.5 mg/day
Lowest and Most Commonly Prescribed Dose2*
Capsule image is not actual size.
- Day 1–Start at 1.5 mg/day
- Day 15–May increase dose to 3 mg/day
Maximum recommended dose is 3 mg/day, depending on clinical response and tolerability.
Following discontinuation of VRAYLAR, the decline in plasma concentrations of active drug and metabolites may not be immediately reflected in patients’ clinical symptoms.1
*Data accessed October 2024.
Flexible and convenient dosing
Bipolar I acute manic or mixed episodes recommended doses1

- Day 1–Start at 1.5 mg/day
- Day 2–Increase dose to 3 mg/day
Further adjustments can be made in 1.5- or 3-mg/day increments, up to a maximum recommended dose of 6 mg/day, depending on clinical response and tolerability.
- In short-term controlled studies, doses above 6 mg/day did not confer increased effectiveness sufficient to outweigh dose-related adverse reactions1
Following discontinuation of VRAYLAR, the decline in plasma concentrations of active drug and metabolites may not be immediately reflected in patients’ clinical symptoms.1
Flexible and convenient dosing
Schizophrenia recommended doses1
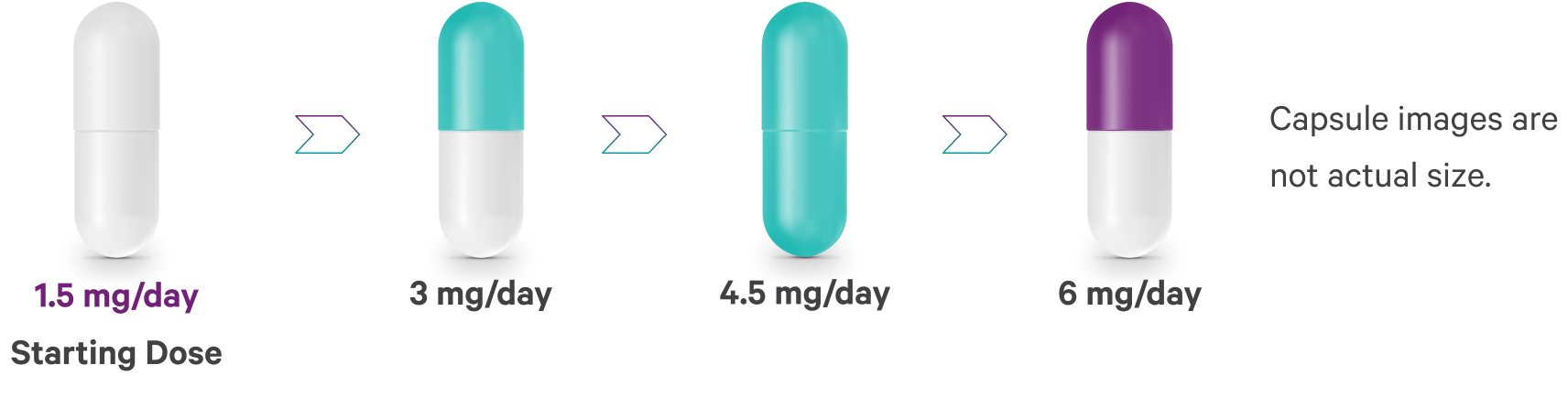
- Day 1–Start at 1.5 mg/day
- Dose can be increased to 3 mg on Day 2
Further adjustments can be made in 1.5- or 3-mg increments, up to a maximum recommended dose of 6 mg/day, depending on clinical response and tolerability.
- In short-term controlled studies, doses above 6 mg/day did not confer increased effectiveness sufficient to outweigh dose-related adverse reactions1
Dosing adjustments
No dosage adjustment required based on1:
- VRAYLAR is not recommended in patients with severe hepatic (Child-Pugh score 10–15) or renal impairment (creatinine clearance <30 mL/min), as it has not been evaluated in these patient populations1
- VRAYLAR can be coadministered with a PPI. It does not affect VRAYLAR exposure at steady state1
PPI=proton pump inhibitor.
Drug interactions
VRAYLAR is primarily metabolized by CYP3A4 and to a lesser extent by CYP2D6.1
CYP3A4 is responsible for the formation and elimination of the major active metabolites in cariprazine.1
Clinically significant drug interactions and dosage adjustments with VRAYLAR1
| Clinical impact | Intervention |
|---|---|
Strong CYP3A4 inhibitors |
Initiating strong CYP3A4 inhibitor while on a stable dose of VRAYLAR
Initiating VRAYLAR therapy while already on a strong CYP3A4 inhibitor
When a strong or moderate CYP3A4 inhibitor is withdrawn, VRAYLAR dosage may need to be increased† |
Moderate CYP3A4 inhibitors |
Initiating moderate CYP3A4 inhibitor while on a stable dose of VRAYLAR
Initiating VRAYLAR while already on a moderate CYP3A4 inhibitor
When a strong or moderate CYP3A4 inhibitor is withdrawn, VRAYLAR dosage may need to be increased† |
CYP3A4 inducers |
Concomitant use of VRAYLAR with a CYP3A4 inducer has not been evaluated and is not recommended |
†Depending upon clinical response and tolerability.
Pharmacokinetics
~3-6 hours to peak plasma concentration1
after 1 dose of VRAYLAR 1.5 mg/day
~7-day half-life1
VRAYLAR plasma concentration decreased by ~50% in approximately 1 week‡
‡After multiple dose administration of VRAYLAR, mean cariprazine and DCAR concentrations reached steady state at around Week 1 to Week 2 and mean DDCAR concentrations approached steady state around Week 4 to Week 8 in a 12-week study. The half-lives based on time to reach steady state were 2 to 4 days for cariprazine, ~1 to 2 days for DCAR, and approximately 1 to 3 weeks for DDCAR. The time to reach steady state for DDCAR was variable across patients, with some not achieving steady state at the end of the 12-week treatment. After discontinuation of VRAYLAR, mean plasma concentrations of cariprazine and DCAR decreased by ~50% in ~1 day, and mean plasma concentration of DDCAR decreased by ~50% 1 week after the last dose. The decline in plasma concentrations of active drug and metabolites may not be immediately reflected in patients’ clinical symptoms.1
BP-I=bipolar I disorder; DCAR=desmethylcariprazine; DDCAR=didesmethylcariprazine; MDD=major depressive disorder.








